Jun 08, 2021
1 min, 56 secs
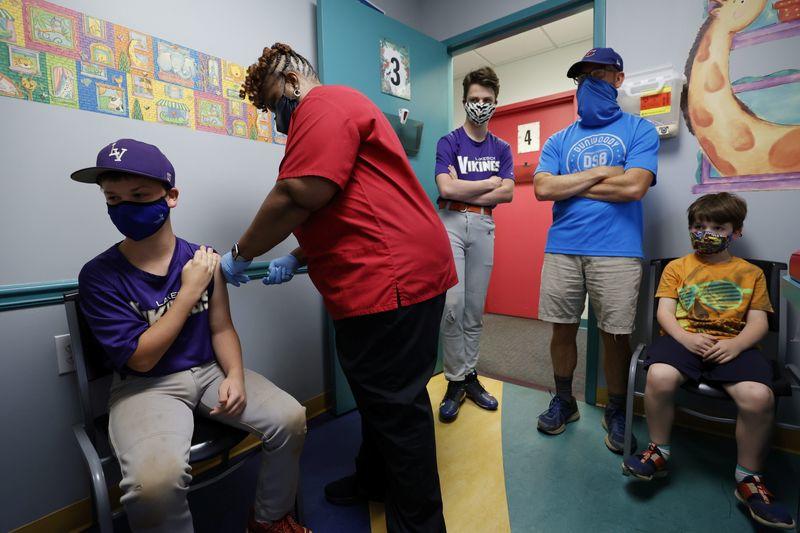
(Reuters) -Pfizer Inc said on Tuesday it will begin testing its COVID-19 vaccine in a bigger group of children under age 12 after selecting a lower dose of the shot in an earlier stage of the trial. . The study will enroll up to 4,500 children at more than 90 clinical sites in the United States, Finland, Poland and Spain, the company said. . Based on safety, tolerability and the immune response generated by 144 children in a phase I study of the two-dose shot, Pfizer said it will test a dose of 10 micrograms in children between 5 and 11 years of age and 3 micrograms for the age group of 6 months to 5. . The vaccine - made by Pfizer and German partner BioNTech SA - has been authorized for use in children as young as 12 in Europe, the United States and Canada. Nearly 7 million teens have received at least one dose of the vaccine in the United States, according to the U.S. Inoculating children and young people is considered a critical step toward reaching "herd immunity" and taming the COVID-19 pandemic. . Still, scientists in the United States and elsewhere are studying the possibility of a link between heart inflammation and mRNA vaccines, particularly in young men. Israel's Health Ministry said last week it had found the small number of myocarditis cases observed mainly in young men who received the Pfizer vaccine there were likely linked to their vaccination.With a smaller study in children complete, Pfizer-BioNTech moves on to a larger COVOD-19 vaccine trial for kids as young as 6 months.Some Sacramento kids will be part of a national clinical trial to test Pfizer's COVID-19 vaccine in those 5 to 11 years old.
Kaiser Permanente Sacramento and Santa Clara locations will join the hospital's Vaccine Research Center in Oakland to enroll 75 kids in the national trial that aims to evaluate 4,600 participants.
Moderna CEO expects COVID-19 vaccine to be available for kids as young as 5 by early fall.J&J’s vaccine was supposed to be an important tool for reaching rural areas and vaccine hesitant Americans because it requires only one shot and has less stringent storage requirements than the two-dose vaccines from Pfizer Inc/BioNTech SE and Moderna Inc.
